| jim.hutchins.name > Jim Hutchins' Resumé > Research Skills | |||||||||||||||||||
Resumé James B. Hutchins |
|||||||||||||||||||
Department of Health Sciences Weber State University Ogden UT 84408-3909 (801) 626-6505 |
|||||||||||||||||||
 |
|||||||||||||||||||
| Research Skills | |||||||||||||||||||
| An up-to-date, mostly complete publication list is always available at PubMed. | |||||||||||||||||||
The theme of my scholarship has been to gain a better understanding of the molecular and cellular changes in the brain as it develops, both under normal conditions and as a result of disease. |
|||||||||||||||||||
As an undergraduate, I worked as part of a team which successfully bred mice for genetic susceptibility to alcohol dependence. My role was to develop measures at the outset of the study which could be reliably used to separate breeding stock into low- and high-dependence groups. This work resulted in one publication. Hutchins J.B., Allen D.L., Cole-Harding L.S. and Wilson J.R. Behavioral and physiological measures for studying ethanol dependence in mice. Pharmac Biochem Behav 15:55-59, 1981. |
|||||||||||||||||||
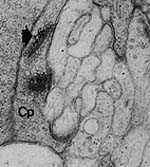 |
My first graduate school project was to study the effects of acetylcholine in chemical signaling between cells of the retina of the tiger salamander. The retina is responsible for receipt and initial processing of light information. This work resulted in two peer-reviewed manuscripts and publication of my master's thesis. Hutchins J.B. Localization of Acetylcholinesterase Activity in the Outer Plexiform Layer of the Larval Tiger Salamander Retina. Masters thesis. Hutchins J.B., Polans A.S. and Werblin F.S. Localization of acetylcholinesterase activity in the outer plexiform layer of the larval tiger salamander retina. Brain Res 292:303-315, 1984. Polans A.S., Hutchins J.B. and Werblin F.S. Muscarinic cholinergic receptors in the retina of the larval tiger salamander. Brain Res 340:355-362, 1985. |
||||||||||||||||||
| A synapse (nerve cell contact) between a cone photoreceptor and processing cells in the salamander retina. | |||||||||||||||||||
A second graduate school project was to determine if acetylcholine played a role in visual processing in the human retina. This work benefited from our unique access to large numbers of human eyes through the Lions Eye Bank in Houston, Texas. I was required to coordinate access to tissue with eye bank technicians and had to be available to process tissue at all times, day or night. This work resulted in six publications in peer-reviewed journals, including one review article which has been cited 27 times by other scientists, and the publication of my doctoral dissertation. Hutchins J.B. Evidence for Acetylcholine as a Neurotransmitter in the Human Retina. Doctoral dissertation. Hutchins J.B. Hollyfield J.G. Autoradiographic identification of muscarinic receptors in human iris smooth muscle. Exp Eye Res 38:515-521, 1984. Hutchins J.B. and Hollyfield J.G. Acetylcholine receptors in the human retina. Invest Ophthalmol Vis Sci 26:1550-1557, 1985. Hutchins J.B. and Hollyfield J.G. Human retinas synthesize and release acetylcholine. J Neurochem 47:81-87, 1986. Hutchins J.B. and Hollyfield J.G. Cholinergic neurons in the human retina. Exp Eye Res 44:363-375, 1987. Hutchins J.B. and Hollyfield J.G. Acetylcholinesterase in the human retina. Brain Res 400:300-311, 1987. Hutchins J.B. Acetylcholine as a neurotransmitter in the vertebrate retina. Exp Eye Res 45:1-38, 1987. |
 |
||||||||||||||||||
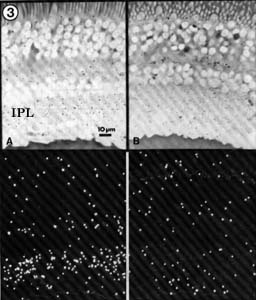
| Human retina stained with 3H-PrBCM, which labels muscarinic acetylcholine receptors. Tiny white "stars" in the lower panels show where radioactive muscarinic receptors are found — notice a band of "stars" in the inner plexiform layer (IPL). | |||||||||||||||||||
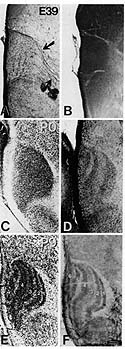 |
My postdoctoral research centered on development of the lateral geniculate nucleus (LGN), a key relay station for visual information processed by the retina. From the LGN, this information is passed along to higher visual centers of the brain including the visual cortex. The LGN initially forms as a solid mass of cells, but during development, the neurons begin to cluster into layers (so-called cytoarchitectonic lamination). The development of the LGN also involves the sorting of retinal input into appropriate locations depending on the part of the visual world represented (a retinotopic map). This work resulted in four publications: Hutchins J.B. and Casagrande V.A. Development of acetylcholinesterase activity in the lateral geniculate nucleus. J Comp Neurol 275:241-253, 1988. Hutchins J.B. and Casagrande V.A. Glial cells develop a laminar pattern before neuronal cells in the lateral geniculate nucleus. Proc Natl Acad Sci 85:8316-8320, 1988. Hutchins J.B. and Casagrande V.A. Vimentin: changes during postnatal brain development. Glia 2:55-66, 1989. Hutchins J.B. and Casagrande V.A. Development of the lateral geniculate nucleus: interactions between retinal afferent, cytoarchitectonic, and glial cell lamination in ferrets and tree shrews. J Comp Neurol 298:113-128, 1990. |
||||||||||||||||||
| The tree shrew lateral geniculate nucleus starts out as a mass of cells that develops layers as the visual system matures. The marker GFAP predicts where these layers will appear before they can be seen by conventional staining methods. | |||||||||||||||||||
Also during my postdoctoral fellowship, I worked to develop biochemical and anatomical methods for use in my ongoing studies. Hutchins J.B. The 'Hawaiian blot': a new Western blotting variant [letter]. BioTechniques 7:248-251, 1989. Casagrande V.A. and Hutchins J.B. Methods for analyzing neuronal connections in mammals. Methods in Neuroscience 3:188-207, 1990. |
 |
||||||||||||||||||
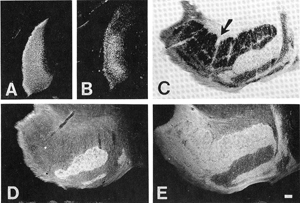
| Part of the ferret lateral geniculate nucleus receives input from the left eye, part from the right. In order to see which part of the LGN receives information from each eye, we use the tracer WGA-HRP (compare A and B from a newborn, and D and E from an adult). | |||||||||||||||||||
 |
My work as principal investigator has focused on two main projects, both of which have been funded. In work supported by the National Eye Institute (of the National Institutes of Health), my colleagues and I studied developmental signals carried by the neurotransmitter acetylcholine. Acetylcholine not only acts as a neurotransmitter in the adult retina, but it plays a critical role in the self-assembly of the retina during development. This work resulted in four publications: Kennedy R.E. and Hutchins J.B. Choline acetyltransferase expression studied with an oligonucleotide probe. Cell Molec Neurobiol 12:309-315, 1992. Hutchins J.B. The development of muscarinic acetylcholine receptors in the ferret retina. Dev Brain Res 82:45-61, 1994. Hutchins J.B., Bernanke J.M. and Jefferson V.E. Acetylcholinesterase in the developing ferret retina. Exp Eye Res 60:113-125, 1995. Hutchins J.B. Functions of acetylcholine in signal processing and development of the mammalian retina. Post-Proceedings of the II Workshop on Cybernetic Vision, IEEE Computer Society Press, ISBN 0-8186-8058-X, 1997. |
||||||||||||||||||
| Four-week old ferret retina labeled with 3H-PrBCM to label muscarinic receptors. | |||||||||||||||||||
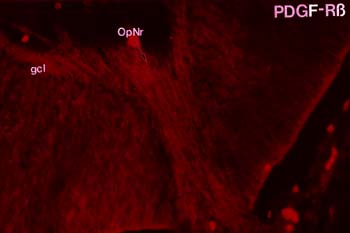
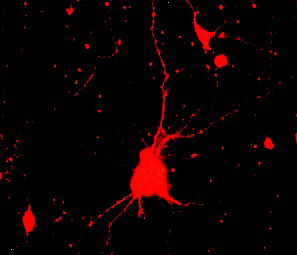
With my co-investigators, I spent a lot of time studying the role of platelet-derived growth factor (PDGF) as a signalling molecule between glia and neurons in the developing brain, particularly the visual system. We moved on to an investigation of the downstream biochemical effects of PDGF on cultured neurons and glia. Xiaorong (Frank) Zhang, working as a graduate student in my laboratory, did some incredibly detailed and elegant experiments to show that actin-binding proteins are modified in PDGF-stimulated neurons. Xiaorong also showed that the mitochondrial enzyme which produces ATP, called F1F0 ATPase, is modified in response to PDGF. This finding has been replicated independently in a separate system. Mark Mattson's laboratory has shown that PDGF exerts neuroprotective effects on cell culture models of ischemia and cytotoxicity; Koji Iihara's lab has demonstrated a neuroprotective effect of PDGF in vivo. Our work with Mark has been highly cited. The Keller et al. article below has been cited 326 times by other scientists in their publications; the Bruce-Keller et al. article has been cited 67 times. Clearly, there is interest in how PDGF works in neurons, either through its effects on the cytoskeleton or on energy production by mitochondria. Hutchins J.B. and Jefferson V.E. Developmental distribution of platelet-derived growth factor in the mouse nervous system. Dev Brain Res 67:121-135, 1992. Hutchins J.B. and Ard M.D. Expression of platelet-derived growth factor and its receptor in rat neuronal and astroglial cultures. Molec Cell Neurosci 4:250-258, 1993. Hutchins J.B. and Zhang X. Platelet-derived growth factor receptors in the developing mouse optic pathway. Visual Neuroscience 11:33-40, 1994. Hutchins J.B. Platelet-derived growth factor receptors of mouse central nervous system cells in vitro. J Comp Neurol 360:59-80, 1995. Zhang F.X., Pan W. and Hutchins J.B. Phosphorylation of F1F0 ATPase |
 |
||||||||||||||||||
 |
|||||||||||||||||||
| Both nerve cell axons growing out of the developing mouse retina and mouse cerebral cortex neurons grown in culture express platelet-derived growth factor |
|||||||||||||||||||
 |
Zhang F.X. and Hutchins J.B. Expression of PDGF receptor Zhang F.X. and Hutchins J.B. Protein phosphorylation in response to PDGF stimulation in cultured neurons and astrocytes. Dev Brain Res 99:216-225, 1997. Keller J.N., Kindy M.S., Holtsberg F.W., St Clair D.K., Yen H.C., Germeyer A., Steiner S.M., Bruce-Keller A.J., Hutchins, J.B. and Mattson M.P. Mitochondrial Mn-SOD prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation and mitochondrial dysfunction. J Neurosci 18: 687-697, 1998. Bruce-Keller A.J., Begley J.G., Fu W., Butterfield D.A., Bredesen D.E., Hutchins J.B., Hensley K. and Mattson M.P. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid Hutchins J.B. and Barger S.W. Why neurons die: cell death in the nervous system. Anatomical Record (New Anatomist) 253(3):79-90, 1998. |
||||||||||||||||||
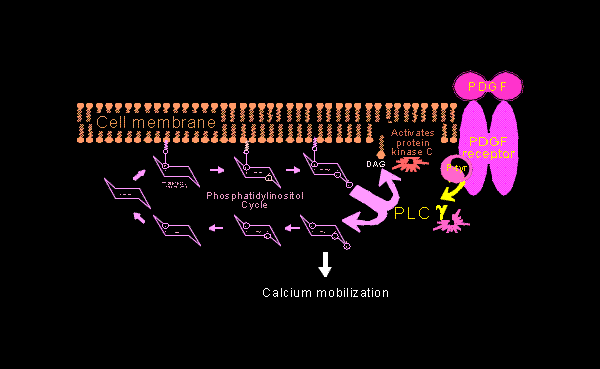
| This is a working model for how PDGF may affect the energy and structure of growing neurons. | |||||||||||||||||||
Working with Zhengwei Cai, I looked at the molecular and cellular biology of hypoxia/ischemia. (Hypoxia is lack of oxygen; ischemia is lack of blood supply, leading to lack of nutrition including oxygen.) We studied the effects on newborn rats and on young adult mice that carry a platelet-derived growth factor (PDGF) transgene. Cai Z., Hutchins J.B. and Rhodes P.G. Intrauterine hypoxia-ischemia alters nitric oxide synthase expression and activity in fetal and neonatal rat brains. Dev Brain Res 109:265-269, 1998. I have published, through the Entrez databases at the National Centers for Biotechnology Information (NCBI), six gene sequences and three expressed sequence tags. When the blood supply is cut off to the fetal and newborn rat's brain, a number of genes are turned on or off in the brain. One of these, which we called "HIC29A", is a RING-type zinc finger protein, and has strong sequence homology to the TRC8 gene involved in cancer of the kidney. No one knows why, but perhaps one day someone will find out. It could be a laboratory artifact, or it could be telling us something about how the brain works. |
 |
||||||||||||||||||
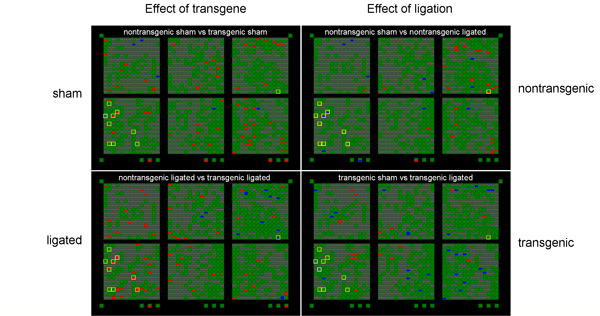 |
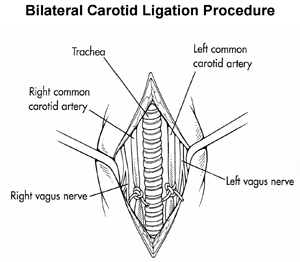
PDGF, applied exogenously, has been shown to protect adult rat brain against hypoxia/ischemia. The hippocampus is especially susceptible to hypoxia/ischemia. Karin Forsberg-Nilsson shared a "knock-in" transgenic mouse line which expresses high levels of PDGF in the brain up to about 4 weeks of age. We bred litters which were half +/- and half -/-. (Survival of +/+ mice is poor.) Hippocampal RNA was isolated from 4 week old mice 24 hours following bilateral carotid artery ligation (“ligated”) or a surgery in which the arteries were exposed but not ligated (“sham”). Both PDGF transgenic mice (+/-) and nontransgenic littermates (-/-) were used and the data compared between the four groups. PDGF transgenic mice may have higher endogenous levels of a mouse protein inhibitor of nitric oxide synthase (mPIN). Transgenic ligated mice have higher mPIN expression levels relative to nontransgenic ligated mice (p<0.05, two-tailed t test). Since we know that nitric oxide is a factor that contributes to neuronal death, we think that higher levels of an inhibitor may contribute to the protective effects of PDGF. |
||||||||||||||||||
| Clontech "Atlas" arrays were used to screen for genes that are up (red) or down (blue) regulated compared to controls. The arrays in this picture are arranged in a grid so that the intersection of transgene (+/-) and sham is at upper left, for example. Yellow boxes show "interesting" genes that were probed further with RT-PCR techniques. One of these was the mouse protein inhibitor of nitric oxide synthase. | |||||||||||||||||||
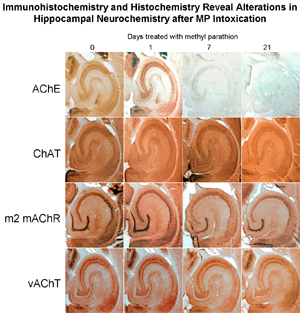
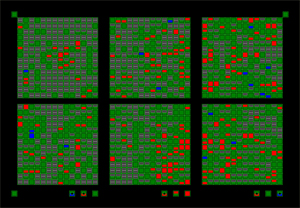
I finished one last project before I closed down the lab. As you can see (if you've read this far), I have extensive experience studying acetylcholine as a neurotransmitter in the brain. Nerve agents, such as sarin, have already been used by terrorists such as the Japanese Aum Shinrikyo cult to cause death and panic; some experts feel as though a nerve agent attack on the United States is inevitable. It is critical to understand how nerve agents such as sarin affect the brain, especially the developing brain. The insecticide methyl parathion resembles nerve agents in the way it acts, and in Mississippi, Michigan and Ohio, children and adults have been sickened — or even killed — by exposure to this pesticide. In China, methyl parathion is used in suicides and attempted suicides. For all these reasons, it is important to know how methyl parathion affects the developing brain. I exposed young mice (from birth to 4 weeks old) and adult mice to methyl parathion by smearing a solution on the back of the neck. (I shaved the fur; the pesticide is readily absorbed through the skin, which is how most people are exposed as well.) Then, I looked at the changes in proteins associated with acetylcholine: acetylcholinesterase (the enzyme which is directly affected by methyl parathion); choline acetyltransferase (an enzyme which makes acetylcholine); the m2 type of muscarinic acetylcholine receptor; and the vesicular acetylcholine transporter (responsible for packaging acetylcholine into vesicles). We also did a survey of changes in gene expression using Atlas arrays. Greenfield R.A., Brown B.R., Hutchins J.B., Iandolo J.J., Jackson R., Slater L.N., and Bronze M.S. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci 323:326-340, 2002. [This work has been cited by 37 other publications to date.] Greenfield R.A., Brown B.R., Hutchins J.B., and Jackson R. Chemical agents as potential weapons of mass destruction. J Okla State Med Assoc 96:309-312, 2003. |
 |
||||||||||||||||||
 |
|||||||||||||||||||
Effects of methyl parathion exposure on the mouose hippocampus. Top: Immunocytochemistry for acetylcholine-related proteins. Bottom: Clontech Atlas gene array assay screening for a wide variety of genes which may be turned on or off after MP exposure. |
|||||||||||||||||||